

Clinical efficacy: XERESE vs acyclovir and placebo
It’s important to remember that XERESE is not a cure—there is no cure for cold sores.1 But, it can help treat the symptoms, reduce severity, and speed healing time in some patients.1
XERESE has a potential for irritation and contact sensitization.1
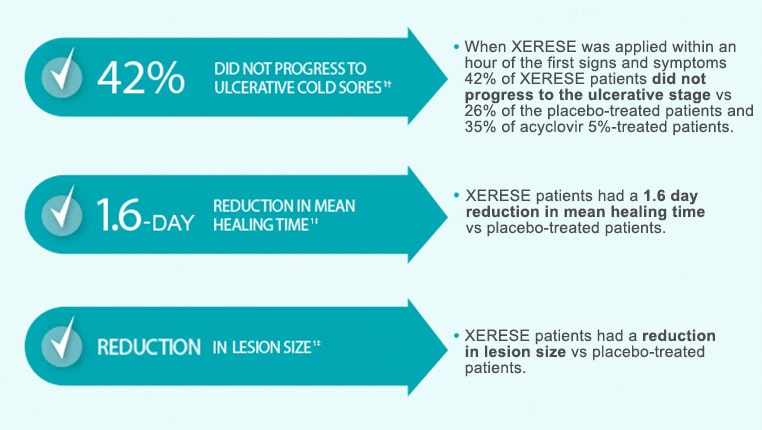
In a double-blind, clinical trial, 1,443 subjects with recurrent labial herpes were randomized to receive XERESE, 5% acyclovir in XERESE vehicle or vehicle alone. Subjects were instructed to initiate treatment within 1 hour of noticing signs or symptoms and continue treatment for 5 days, with application of study medication 5 times per day.
next: ROLE OF INFLAMMATION
†Compared with 26% on placebo. Randomized study of 1443 treated patients who received XERESE within 1 hour of noticing signs or symptoms.
‡Compared with placebo. Randomized study of 1443 treated patients who received XERESE within 1 hour of noticing signs or symptoms.
REFERENCE: 1. XERESE® (acyclovir and hydrocortisone) cream 5%/1% Prescribing Information. August 2020.
Important safety information
- XERESE (acyclovir and hydrocortisone) cream 5%/1% is intended for cutaneous use only, on the lips and around the mouth. XERESE should not be used in the eye, inside the mouth or nose, or on the genitals.
- Systemic exposure to acyclovir and hydrocortisone following topical administration is minimal. However, caution should be exercised when XERESE is administered to women who are pregnant or nursing. The benefit of XERESE has not been adequately assessed in immunocompromised patients.
- XERESE has a potential for irritation and contact sensitization.
- In clinical trials, the most common adverse reactions in the area of the application site included drying or flaking of the skin; burning or tingling following application; erythema; pigmentation changes; application site reaction including signs and symptoms of inflammation. Each event occurred in less than 1% of patients.
- Patients should be encouraged to seek medical advice when a cold sore fails to heal within 2 weeks.
INDICATION
XERESE® (acyclovir and hydrocortisone) cream 5%/1% is indicated for the early treatment of recurrent herpes labialis (cold sores) to reduce the likelihood of ulcerative cold sores and to shorten the lesion healing time in patients 6 years of age and older.
To report SUSPECTED ADVERSE REACTIONS, contact Bausch Health LLC at 1-800-321-4576 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Click here for full Prescribing Information.

